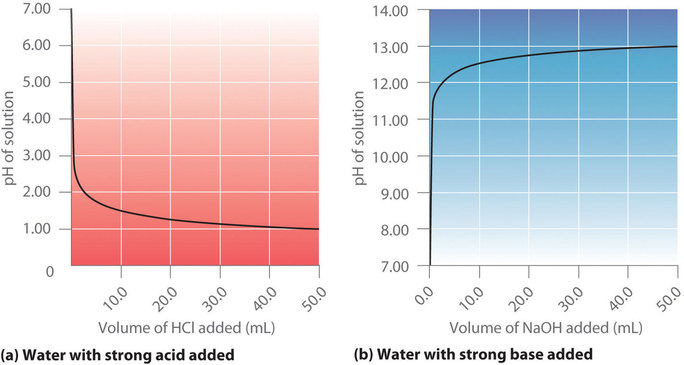
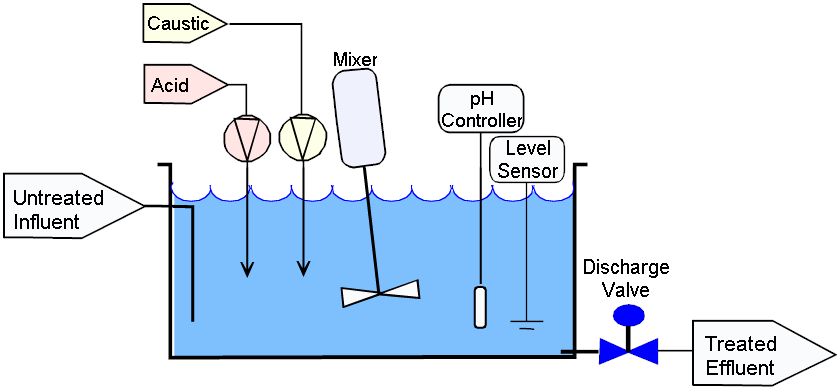
SOLVED: What is neutralization? Write an equation for the neutralization of HCI by NaOH: Calculate the Ht ion concentration of orange juice with pH = 3.35? What is titration? Why is it
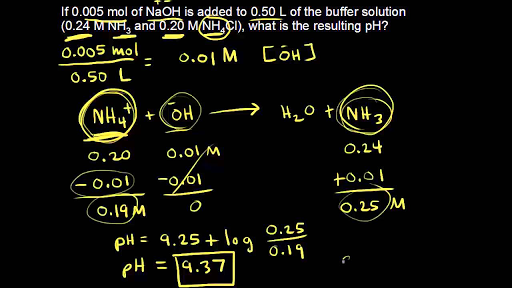
What is the pH of a buffer that has 0.100 moles HC2H3O2 and 0.100 moles NaC2H3O2 in 1.00 l that has 0.010 moles NaOH added to it? - Quora

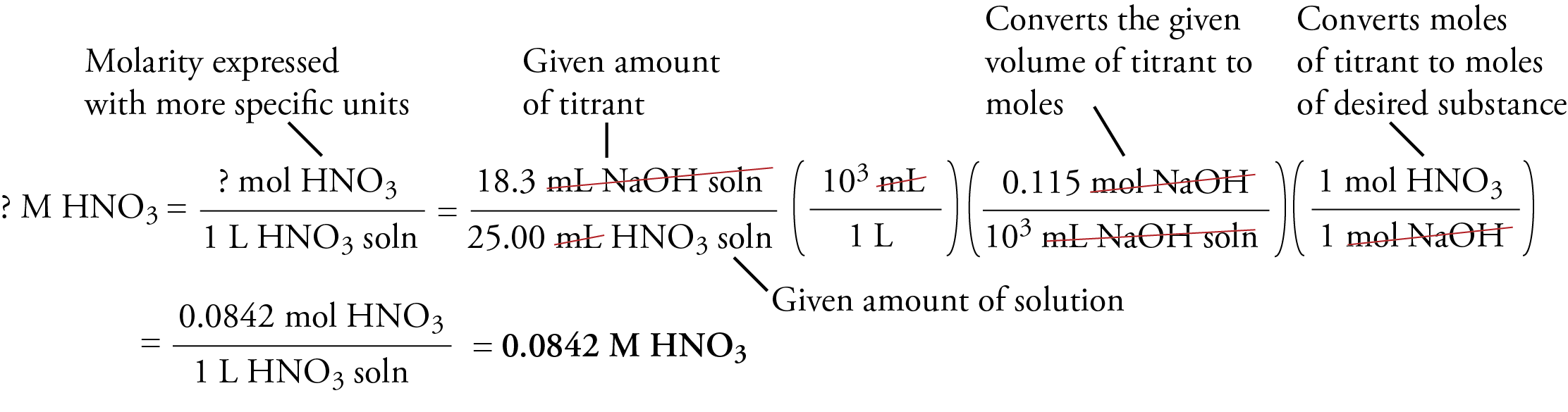
Calculate the concentration of HCl acid if 50 ml of HCl is required to neutralize 25 ml of 1 M NaOH in acid base titration.

Calculate the concentration of HCl acid if 50 ml of HCl is required to neutralize 25 ml of 1 M NaOH in acid base titration.
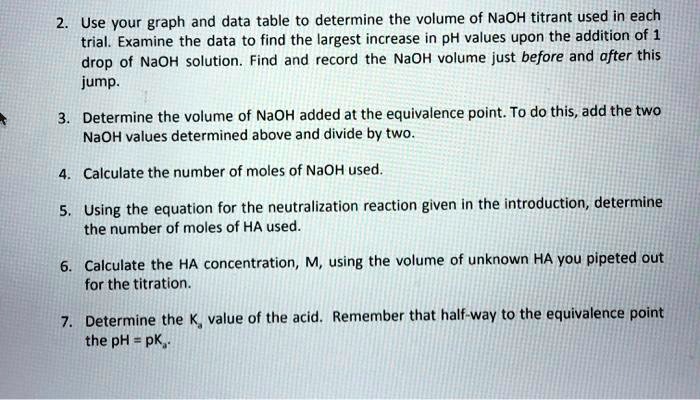
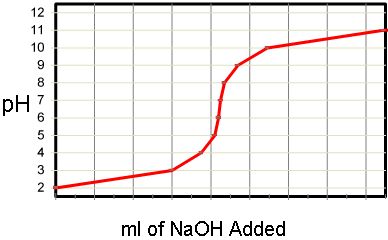
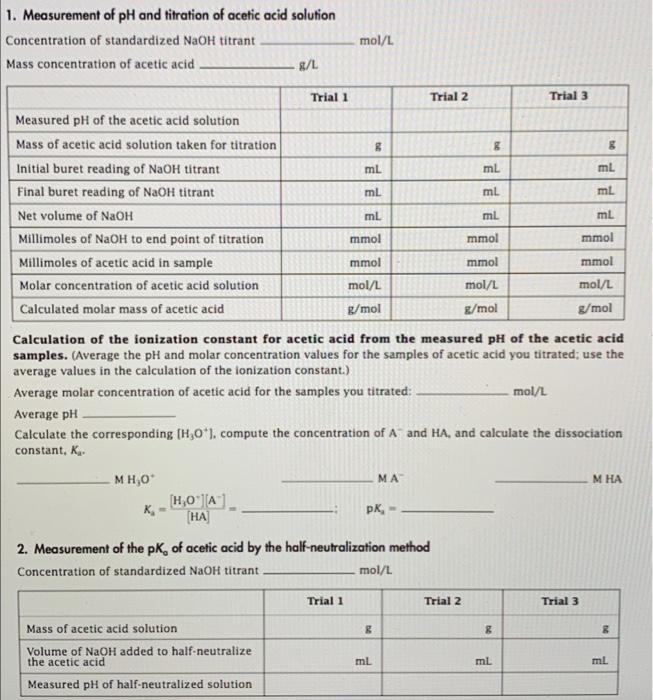
SOLVED: Use vour graph and data table to determine the volume of NaOH titrant used in each trial. Examine the data to find the largest increase in pH values upon the addition