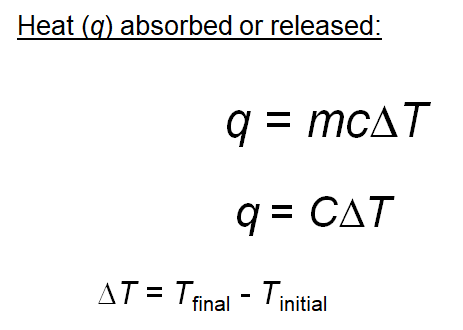
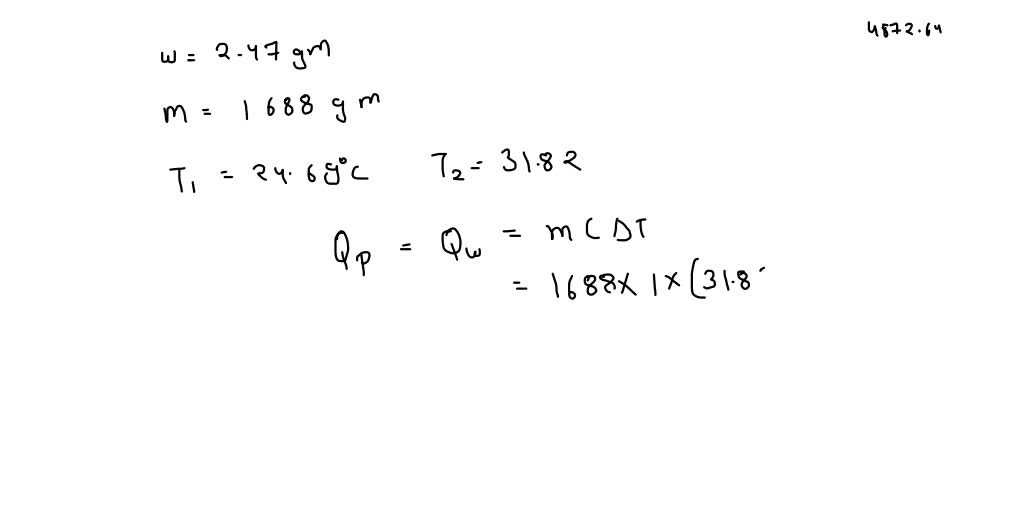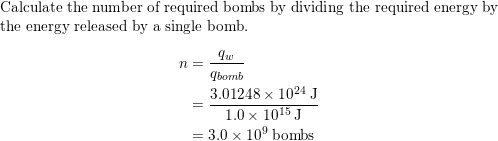For complete combustion of ethanol, C2H5OH(l) + 3O2(g)→ 2CO2(g) + 3H2O(l) , the amount of heat produced as measured in bomb calorimeter is 1364.47 kJ/mol at 25^C . Assuming ideally, the enthalpy

An explosion of atomic bomb releases 7.6 × 10^13J energy. If 200 MeV energy is released on fission of one ^235U atom, then the number of uranium atoms undergoing fission and the