The Influence of Impurities on the Dehydration and Conversion Process of Calcium Sulfate Dihydrate to α-Calcium Sulfate Hemihydrate in the Two-Step Wet-Process Phosphoric Acid Production | ACS Sustainable Chemistry & Engineering
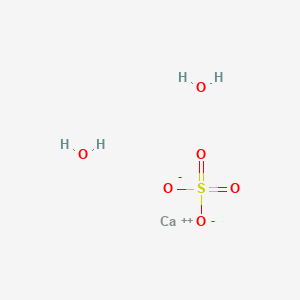
Membranes | Free Full-Text | Gypsum (CaSO4·2H2O) Scaling on Polybenzimidazole and Cellulose Acetate Hollow Fiber Membranes under Forward Osmosis

Dehydration Pathways of Gypsum and the Rehydration Mechanism of Soluble Anhydrite γ-CaSO4 | ACS Omega

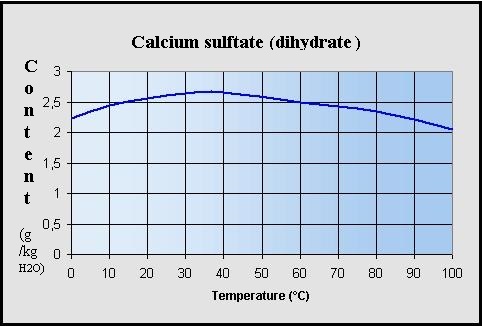
Reaction control of CaSO4 during hydration/dehydration repetition for chemical heat pump system - ScienceDirect

Dehydration Pathways of Gypsum and the Rehydration Mechanism of Soluble Anhydrite γ-CaSO4 | ACS Omega

Dehydration Pathways of Gypsum and the Rehydration Mechanism of Soluble Anhydrite γ-CaSO4 | ACS Omega
A crystallographic study of the low-temperature dehydration products of gypsum, CaSOa ' 2H2Oz hemihydrate CaSOr ' 0.50H2O, and 1

Experimental Study of Hydration/Dehydration Behaviors of Metal Sulfates M2(SO4)3 (M = Sc, Yb, Y, Dy, Al, Ga, Fe, In) in Search of New Low-Temperature Thermochemical Heat Storage Materials | ACS Omega

Dehydration Pathways of Gypsum and the Rehydration Mechanism of Soluble Anhydrite γ-CaSO4 | ACS Omega

Dehydration Pathways of Gypsum and the Rehydration Mechanism of Soluble Anhydrite γ-CaSO4 | ACS Omega

Structures of a) gypsum, b) hemihydrate, and c) insoluble anhydrite... | Download Scientific Diagram
A crystallographic study of the low-temperature dehydration products of gypsum, CaSOa ' 2H2Oz hemihydrate CaSOr ' 0.50H2O, and 1

Figure 1 from In Situ Raman Spectroscopic Study of Gypsum (CaSO4·2H2O) and Epsomite (MgSO4·7H2O) Dehydration Utilizing an Ultrasonic Levitator. | Semantic Scholar

Dehydration Pathways of Gypsum and the Rehydration Mechanism of Soluble Anhydrite γ-CaSO4 | ACS Omega

Thermodynamic Modeling of Calcium Sulfate Hydrates in the CaSO4–H2O System from 273.15 to 473.15 K with Extension to 548.15 K | Journal of Chemical & Engineering Data

Dehydration Pathways of Gypsum and the Rehydration Mechanism of Soluble Anhydrite γ-CaSO4 | ACS Omega

Determination and Analysis of the Solubility of CaSO4·2H2O and α-CaSO4·0.5H2O in Formamide Aqueous Solutions at T = 303.15–363.15 K | Journal of Chemical & Engineering Data

Experimental study of the dehydration reactions gypsum-bassanite and bassanite-anhydrite at high pressure: indication of anomalous behavior of H(2)O at high pressure in the temperature range of 50-300 degrees C. | Semantic Scholar