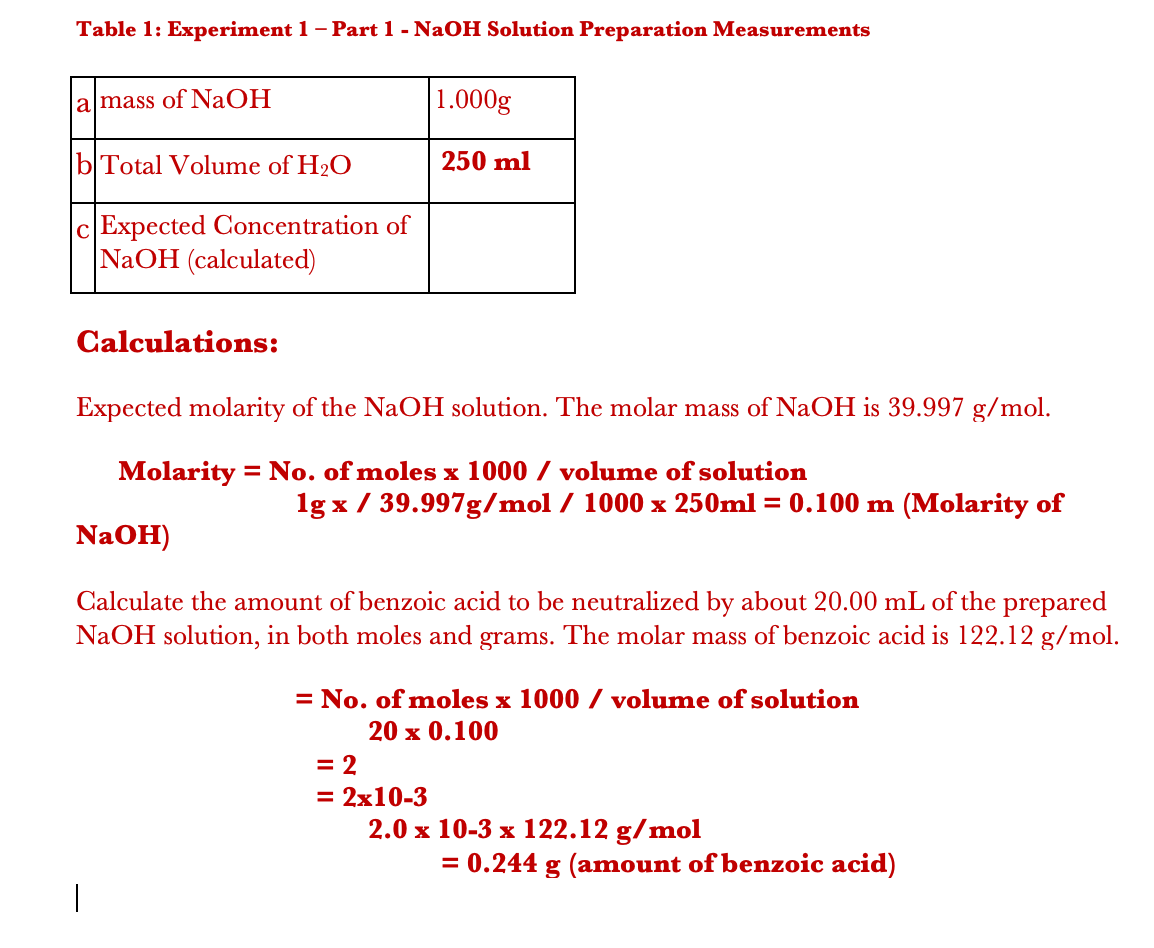
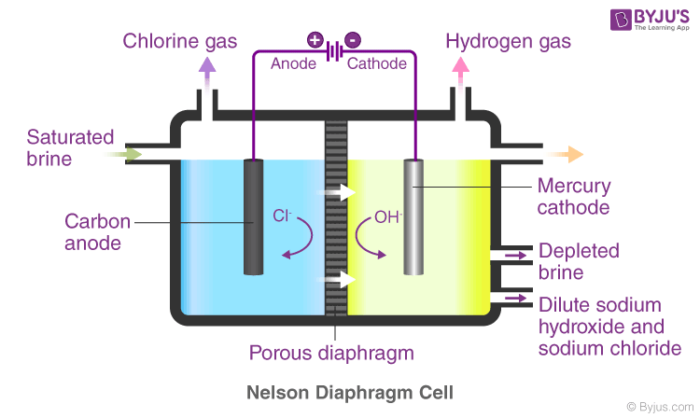
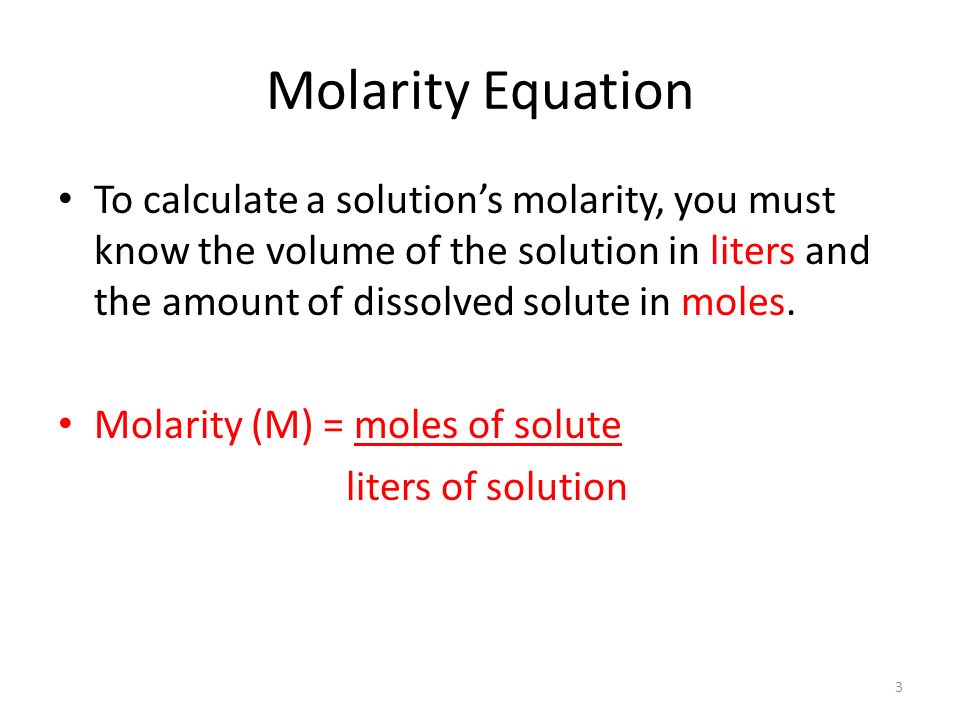
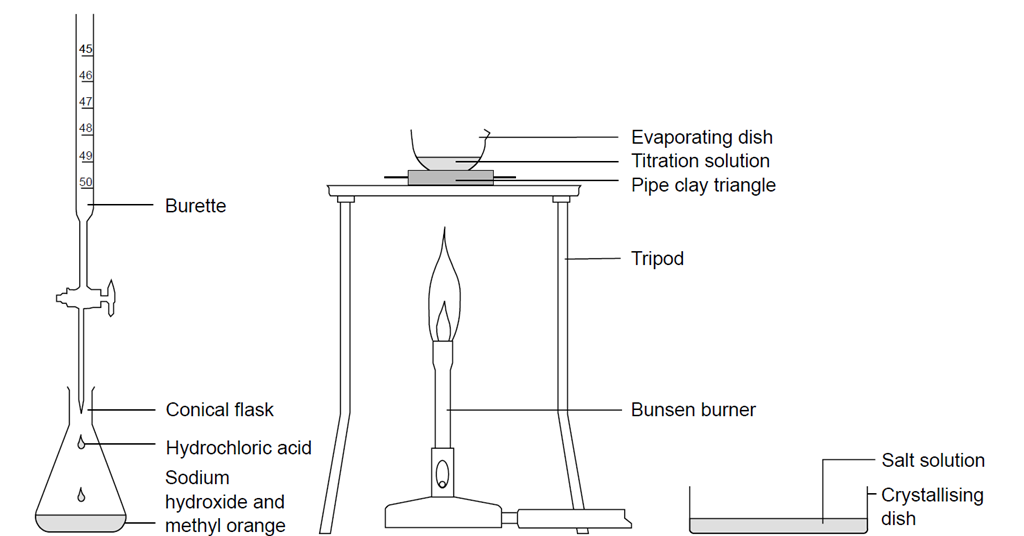
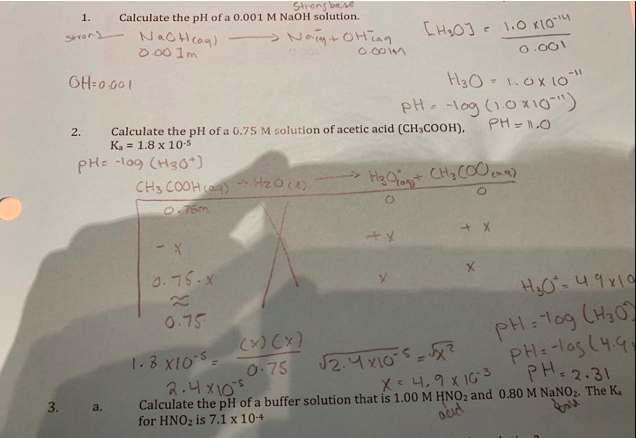
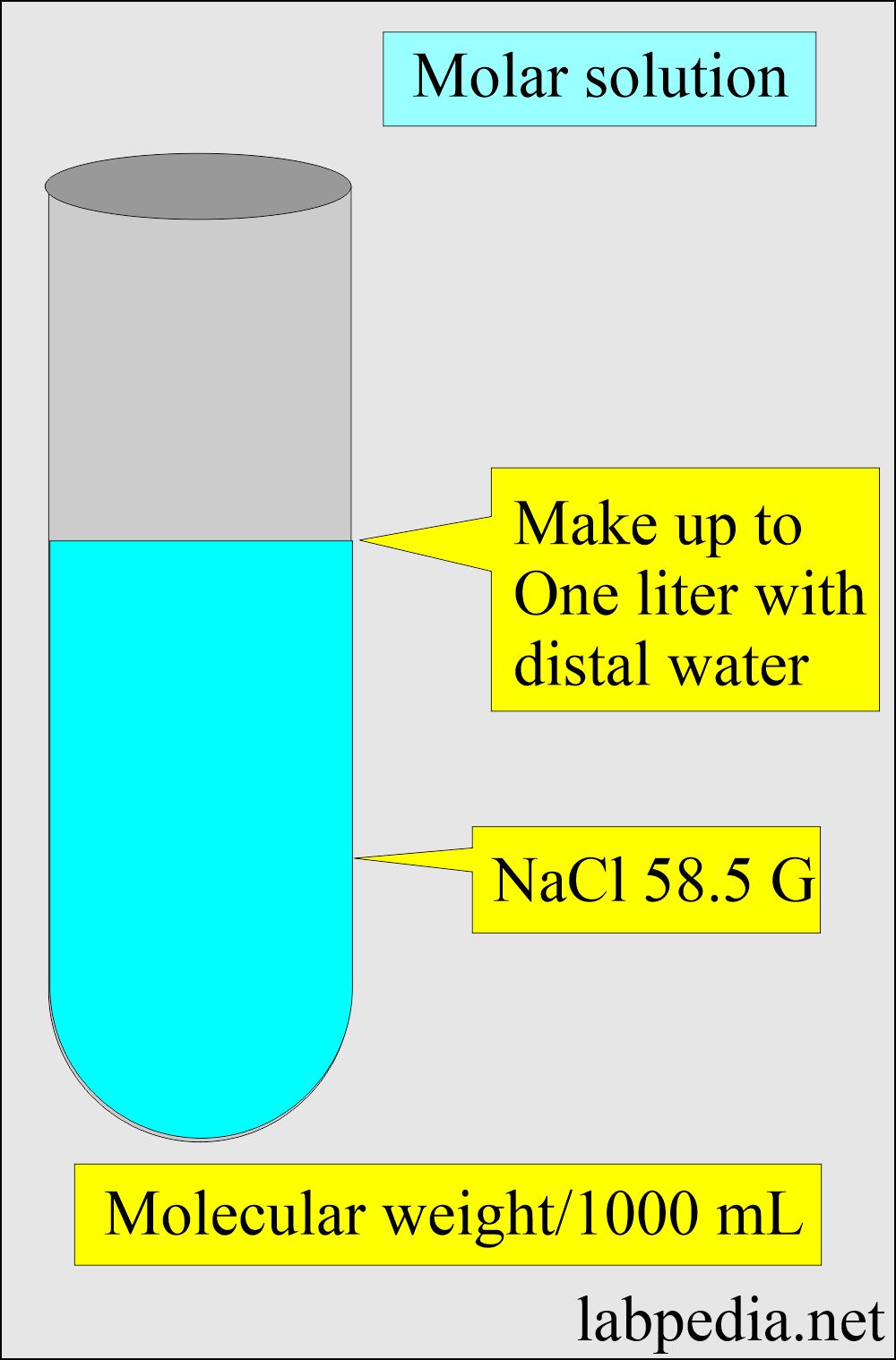
4 g NaOH is added in 100 mL of 0.5 M NaOH solution and solution was made 1 L with addition of water 20 mL of above solution can:

Calculate the molarity of NaOH in the solution prepared by dissolving its 4 g in enough water to... - YouTube

SOLVED: Calculate the pH of a 6.7 X 10-2 M NaOH solution. ( a strong base) ( Your answer should have 2 digits after the decimal)

A sodium hydroxide solution containing 40% by weight of pure NaOH has a specific gravity of 1.5. What volume of this solution will be required in the preparation of 500ml of a

Calculate molality of 2 molar NaOH solution which is 10% by weight - Chemistry - Solutions - 12944633 | Meritnation.com

Calculate the molarity of NaOH in the solution prepared by dissolving its 4g in enough water ..... - YouTube
![Calculate the mole fraction of NaOH in 10% w/w aqueous solution. [At. MassH=1, O=16, Na=23u] - Brainly.in Calculate the mole fraction of NaOH in 10% w/w aqueous solution. [At. MassH=1, O=16, Na=23u] - Brainly.in](https://hi-static.z-dn.net/files/da5/28dabad35d0225715bc998463bc22ff5.jpg)
:max_bytes(150000):strip_icc()/prepare-sodium-hydroxide-or-naoh-solution-608150_FINAL-696b52d6f90b4b1383ec8f95db73a1f3.png)