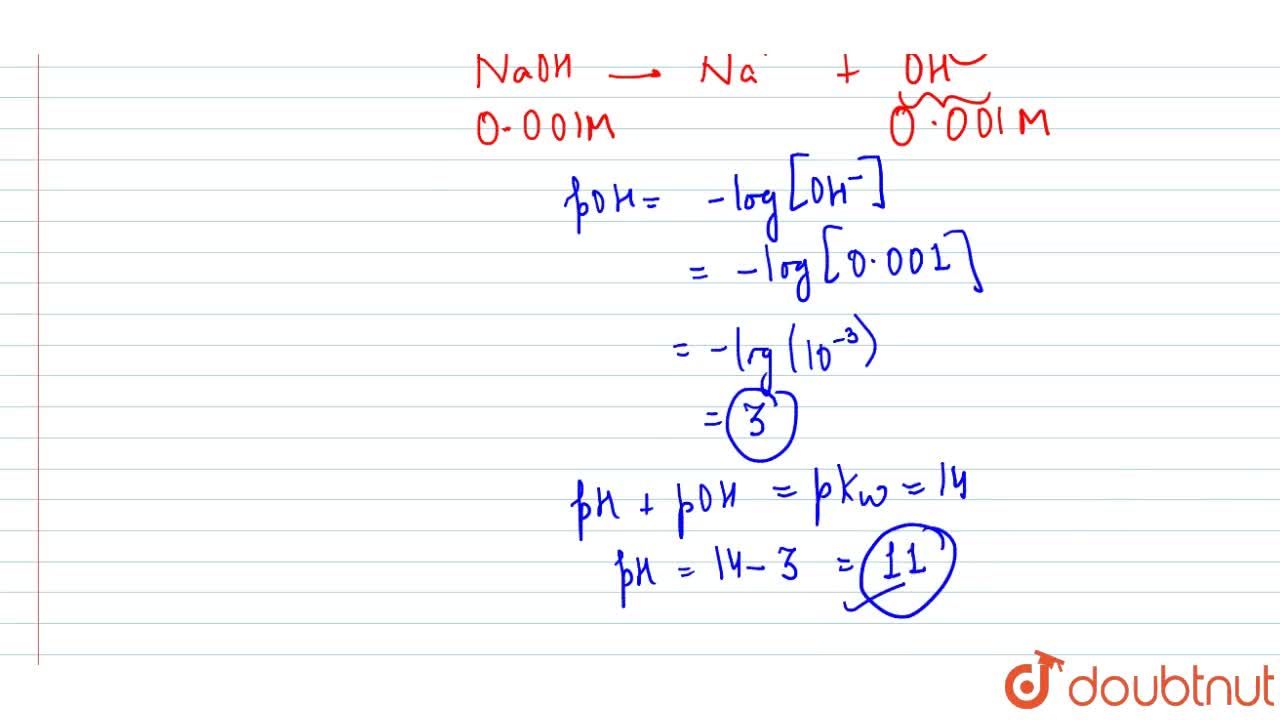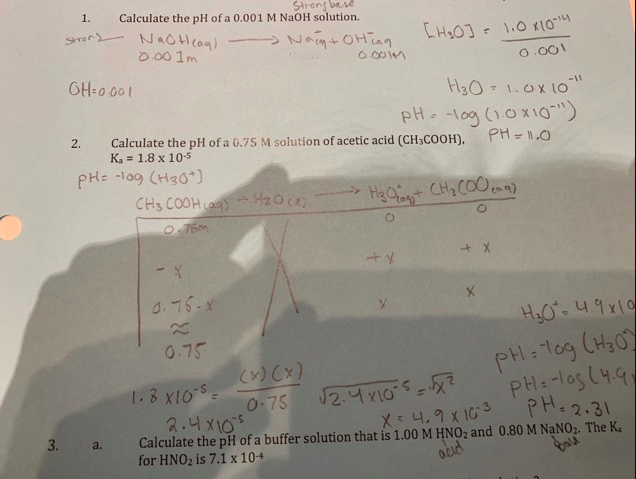Calculate pH for : (a) 0.001 N NaOH, (b) 0.01 N Ca(OH)2 - Sarthaks eConnect | Largest Online Education Community

Topic 18- Acids and bases 18.1 Calculations involving acids and bases 18.2 Buffer solutions 18.3 Salt hydrolysis 18.4 Acid-base titrations 18.5 Indicators. - ppt video online download
Calculate pH for : (a) 0.001 N NaOH, (b) 0.01 N Ca(OH)2 - Sarthaks eConnect | Largest Online Education Community

Calculate pH for: (a) `0.001 NaOH`, (b) `0.01N Ca(OH)_(2)`, (c ) `0.01M Ca(OH)_(2)`, (d) `10^(-8 - YouTube
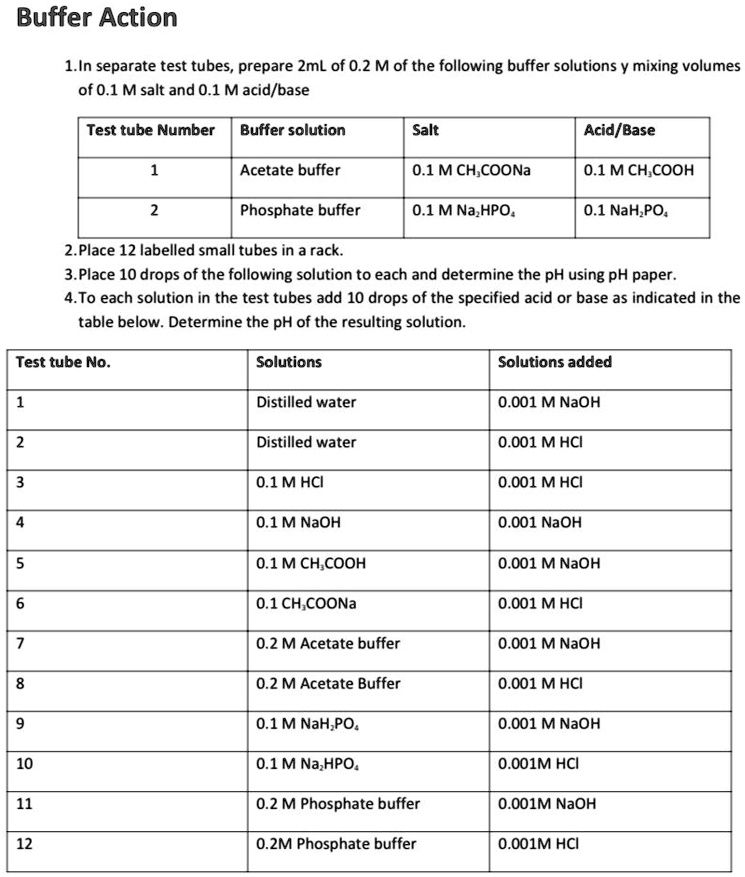
SOLVED: Buffer Action 1.In separate test tubes, prepare ZmL of 0.2 M of the following buffer solutions mixing volumes of 0.1 M salt and 0.1 Macid/base Test tube Number Buffer solution Salt

Assuming complete dissociation, calculate the pH of the following solutions:(a) 0.003 M HCl (b) 0.005 M NaOH (c) 0.002 M HBr (d) 0.002 M KOH
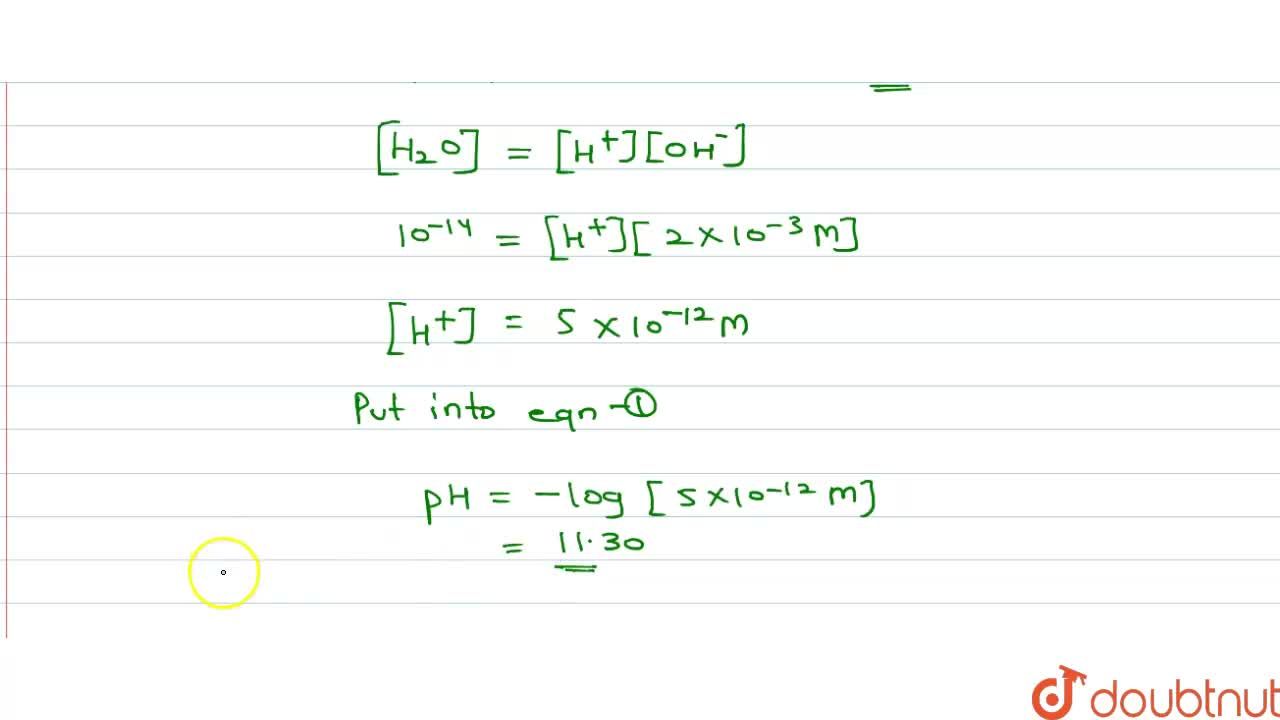
Assuming complete dissociation, calculate the pH of the following solutions, a. 0.003 M HCl, b. 0.005 M NaOH, c. 0.002 M HBr, d. 0.002 M KOH
What is the pH of the resulting solution when the equal volume of 0.1M Noah and 0.01M HCL are mixed? - Quora
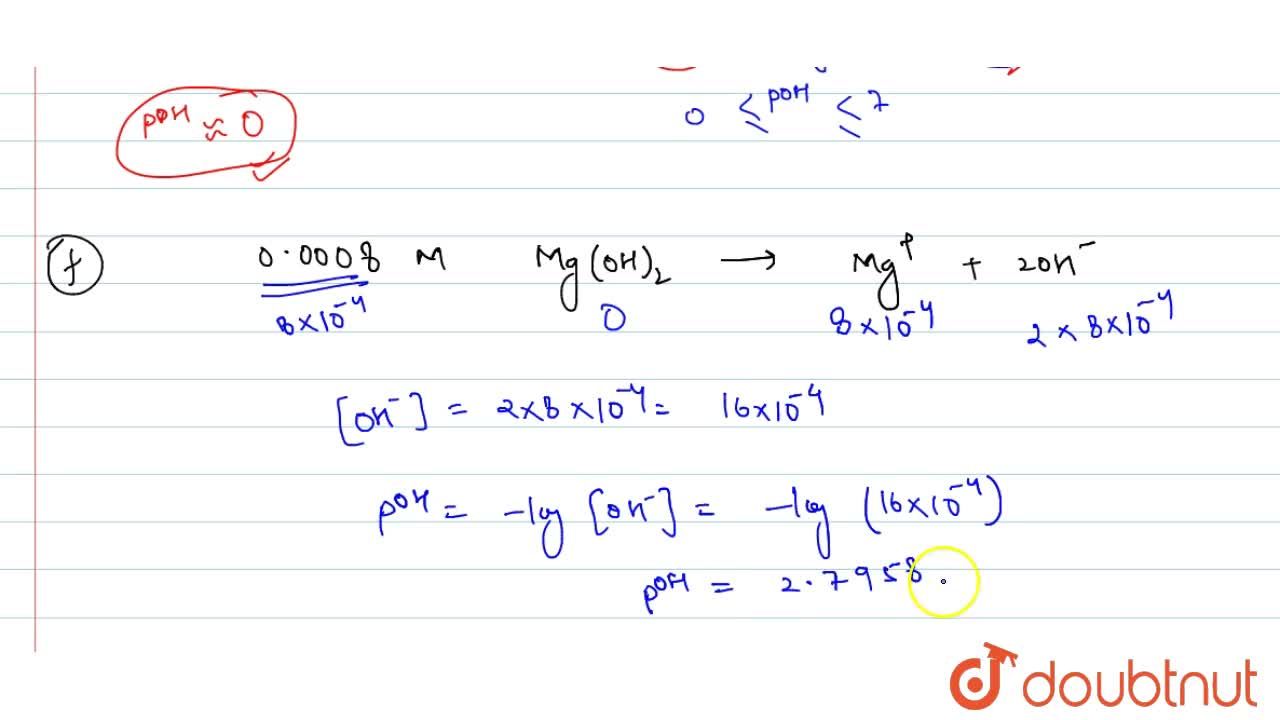
Calculate pH for: (a) 0.001 NaOH, (b) 0.01N Ca(OH)(2), (c ) 0.01M Ca(OH)(2), (d) 10^(-8)M NaOH, (e ) 10^(2)MNaOH, (f) 0.0008MMg(OH)(2) Assume complete ionisation of each.
![SOLVED: Q: What are the [H+], [OH-], pH and pOH of a 0.002M solution of HNO3? Nitric acid v strong acid Completely ionizes in water HNO3 7 Ht + NO3 pH + SOLVED: Q: What are the [H+], [OH-], pH and pOH of a 0.002M solution of HNO3? Nitric acid v strong acid Completely ionizes in water HNO3 7 Ht + NO3 pH +](https://cdn.numerade.com/ask_images/096d2e96c5f04ffb9944004e8f1d5fba.jpg)
SOLVED: Q: What are the [H+], [OH-], pH and pOH of a 0.002M solution of HNO3? Nitric acid v strong acid Completely ionizes in water HNO3 7 Ht + NO3 pH +
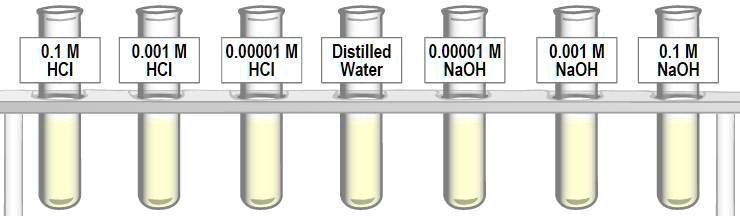
SOLVED: 'What are the pH of these solutions? 0.1 M HCI 0.001 M HCI 0.00001 M HCI Distilled Water 0.00001 M NaOH 0.001 M NaOH 0.1 M NaOH'